In an era where clean energy is not just an option but a necessity, hydrogen stands out as a cornerstone in the quest for sustainable power generation. The leaps in technological advancements and shifts in regulatory landscapes have paved the way for hydrogen to become a focal point in discussions about the future of energy. This comprehensive article aims to demystify hydrogen technology, focusing on its application in internal combustion engines and fuel cells for electric power generation, tailored for the analytical minds of electrical engineers.
Diving into the atomic structure, hydrogen, denoted as H or H2, is the simplest and first element on the periodic table, often existing as a diatomic molecule (H2) due to its unstable atomic form. This dual atomic bonding (Figure 1 and Figure 2) renders hydrogen stable and suitable for various applications including energy production.
The Appeal of Hydrogen in Energy Generation
Three pivotal attributes elevate hydrogen as a prime candidate for electric power generation:
Exceptional Energy Carrier: Hydrogen’s remarkable energy content per weight surpasses that of traditional fuels like diesel and gasoline, making it an efficient energy carrier.
Clean Fuel: The interaction between hydrogen and oxygen in fuel cells or engines produces only water and energy, presenting a clean, emission-free power source.
Abundance: Despite hydrogen’s widespread availability in compounds, its extraction does require energy, a fact overshadowed by the energy and environmental benefits it offers.
Hydrogen Production Techniques.
The U.S. annually produces about 10 million metric tons of hydrogen, primarily for industries like ammonia production and petroleum refining. However, its reach is expanding into electric power generation and transportation, employing processes such as:
Biological: Utilizing microorganisms to produce hydrogen.
Electrolytic: Employing electricity to split water into hydrogen and oxygen, generating “green hydrogen” if sourced from renewables.
Photolytic: Using solar energy for water-splitting.
Thermochemical: Extracting hydrogen from organic materials using chemical reactions and heat, notably through steam methane reforming (SMR).
Each method comes with its distinct operational principles and environmental implications, paving varying pathways to harness hydrogen’s power.
Hydrogen Categorization
Hydrogen’s environmental footprint depends on its production source, categorizing it into green, blue, grey, brown, pink, and yellow hydrogen, each reflecting the production process and its climate impact.
Innovative Storage Solutions
Despite hydrogen’s lightweight nature and high energy content, its storage and transport require innovative approaches to enhance its density and manage its flammability. Techniques range from high-pressure gaseous storage and cryogenic liquid storage to solid-state storage, addressing the volumetric efficiency challenges.
The Role of Hydrogen in Internal Combustion Engines
The incorporation of hydrogen fuel into the realm of internal combustion (IC) engines signals a pivotal shift toward reducing the environmental footprint of traditional fuel-based mobility and power generation. This transformative move, however, comes with its set of technical challenges and considerations that require a deeper exploration to fully comprehend the implications and potential of hydrogen’s role in IC engines.
Hydrogen’s Potential in Reducing Emissions
One of the most compelling arguments for the integration of hydrogen into IC engine operations is its potential to significantly curb emissions that contribute to air pollution and climate change. When hydrogen combusts, it primarily produces water vapor and energy, devoid of carbon emissions. This characteristic presents an opportunity for drastic reductions in carbon monoxide (CO), particulate matter (PM), and unburnt hydrocarbons (HC), which are prevalent by-products of conventional fossil fuels.
The Challenges of Pure Hydrogen Utilization
Fully transitioning to 100% hydrogen fuel within IC engines demands substantial design overhauls. Due to hydrogen’s distinct combustion characteristics, including its wide flammability range and higher flame speed compared to traditional fuels, engine components and fuel delivery systems must be re-engineered to accommodate these differences. These modifications aim to harness hydrogen’s energy effectively while ensuring operational safety and durability under varying conditions.
Navigating the Dual-Fuel Strategy
A pragmatic approach to mitigating the immediate challenges of hydrogen adoption is the strategy of blending hydrogen with traditional fuels for use in spark-ignited (SI) engines. This technique leverages hydrogen’s clean-burning properties to enhance combustion efficiency and reduce harmful emissions. As hydrogen is introduced into the fuel mix, it aids in more complete combustion, reducing CO, PM, and HC emissions. However, this advantage is not without its trade-offs.
Addressing Increased NOx Emissions
One of the paramount challenges in hydrogen-fueled or hydrogen-blended IC engines is the management of nitrogen oxide (NOx) emissions. The high combustion temperatures fostered by hydrogen elevate the production of NOx, a potent pollutant that poses risks to both human health and the environment. Addressing this issue requires innovative solutions such as advanced exhaust after-treatment systems or adjustments to the combustion process itself to limit NOx formation without compromising engine efficiency and performance.
Engine Performance Considerations
The integration of hydrogen, whether in pure form or as a blend, has nuanced effects on key performance metrics of IC engines, including brake horsepower (BHP) and brake thermal efficiency (BTE). Hydrogen’s low volumetric energy density compared to gasoline or diesel fuels can lead to reductions in BHP, primarily due to decreased volumetric efficiency as the engine accommodates the gaseous fuel. Conversely, the high autoignition temperature of hydrogen necessitates advancements in ignition systems and combustion control to optimize performance and efficiency. Adapting to these changes while maintaining or enhancing BTE is a focal area of ongoing research, aiming to capitalize on hydrogen’s potential without compromising the power output and operational efficiency that end users expect from their engines.
The path to incorporating hydrogen into internal combustion engines is riddled with challenges that require innovative engineering solutions and a commitment to sustainable energy progress. As environmental regulations become stricter and the demand for cleaner transportation and power generation methods grows, the exploration and development of hydrogen-fueled engines will undoubtedly continue. With each advancement, the vision of a low-emission, high-efficiency future powered by hydrogen inches closer to reality, heralding a new era for internal combustion engine technology.
Electrifying Power Generation with Hydrogen Fuel Cells
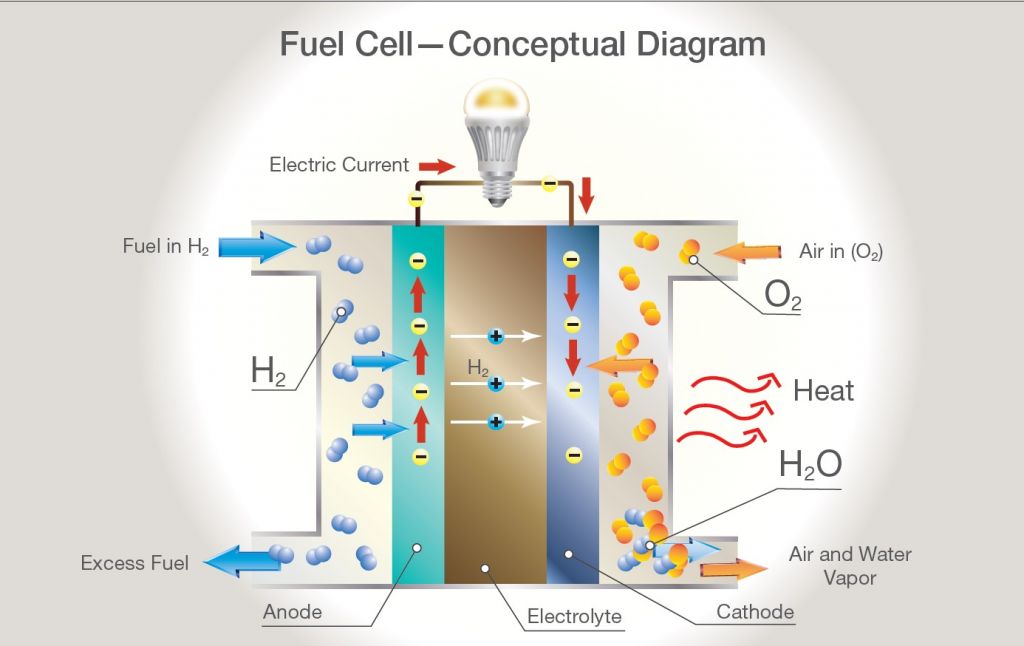
Electrifying Power Generation with Hydrogen Fuel Cells: A Deep Dive
Hydrogen fuel cells are poised at the brink of revolutionizing the landscape of electric power generation. Serving as an electrochemical powerhouse, these devices convert hydrogen and oxygen into electricity through processes that produce only water and heat as by-products, showcasing an unparalleled leap towards clean and efficient energy production.
The Essence of Fuel Cells
At their core, hydrogen fuel cells operate on a simple principle: they combine hydrogen with oxygen to produce electricity, water, and heat. This process, inherently clean and efficient, starkly contrasts with traditional power production methods that rely on combustion and are thus marred by pollution and inefficiency.
The Spectrum of Hydrogen Fuel Cells
Hydrogen fuel cells are not a one-size-fits-all solution; they are a diverse array of technologies each optimized for specific applications and operational parameters. This diversity is broadly categorized into five primary technologies:
Polymer Electrolyte Membrane (PEM) Fuel Cells: These fuel cells, sometimes referred to as Proton Exchange Membrane Fuel Cells, are characterized by their acid membrane electrolytes and the use of a solid polymer. Utilizing porous carbon electrodes infused with a platinum or platinum alloy catalyst, PEM fuel cells excel in low operating temperatures (around 80°C) and rapid start-up times, making them ideal for transportation and backup power applications. Their electrical efficiency ranges between 45% and 65%.
Solid Oxide Fuel Cells (SOFC): SOFC technology utilizes a hard, non-porous ceramic compound as an electrolyte and operates at very high temperatures (up to 1000°C). This high operational temperature enables internal reforming, where methane and light hydrocarbons are converted to hydrogen within the fuel cell itself. SOFCs are celebrated for their high electrical efficiency (up to 85% in combined heat and power applications) and their flexibility in fuel choice, including natural gas and biogas.
Alkaline Fuel Cells (AFC): Being among the earliest developed fuel cell technologies, AFCs use a solution of potassium hydroxide in water as the electrolyte and can operate with a variety of non-precious metal catalysts. They require pure hydrogen and oxygen to function efficiently and are particularly sensitive to impurities such as CO2, which can significantly impact their performance and lifespan. AFCs are commonly used in space applications due to their high electrical efficiency (around 60%).
Phosphoric Acid Fuel Cells (PAFC): Utilizing liquid phosphoric acid as an electrolyte, PAFCs operate at a moderate temperature range of 150–200°C. They are one of the more mature fuel cell technologies, offering an electrical efficiency of approximately 85% when applied in combined heat and power systems. However, the use of pure hydrogen as a fuel and the high cost of platinum catalysts pose challenges to their widespread adoption.
Molten Carbonate Fuel Cells (MCFC): MCFC technology leverages a molten carbonate salt mixture suspended in a porous, chemically inert ceramic matrix as its electrolyte. Operating at high temperatures of 600°–700°C, MCFCs can reform light hydrocarbons to hydrogen directly within the cell. This unique feature, coupled with their ability to operate on various hydrogen-rich fuels and their high electrical efficiency, positions MCFCs favorably for large-scale industrial applications.
Hydrogen fuel cells hold the promise of transforming the way we generate and consume energy. Their ability to provide clean, efficient, and scalable power solutions aligns with global efforts to transition to sustainable energy systems. As technological advancements continue to address current limitations, such as reducing the cost of catalyst materials and improving durability, the role of hydrogen fuel cells in the energy sector is set to expand, encapsulating everything from portable power and transportation to grid support and large-scale industrial applications. The journey toward a cleaner energy future is well underway, with hydrogen fuel cells leading the charge.
The journey towards a hydrogen-fueled future is laden with both opportunities and challenges. For electrical engineers venturing into the realm of clean energy, understanding the nuances of hydrogen technology—from production and storage methods to its application in power generation—is critical. The conversion of hydrogen into a pivotal energy source for internal combustion engines and fuel cell technologies underscores the potential of this elemental resource in crafting a sustainable energy landscape. As developments unfold, keeping abreast of hydrogen’s evolving role will equip electrical engineers with the knowledge to innovate and drive the clean energy revolution forward.


